Why Zilxi?
START GETTING ON TOP OF ROSACEA
ABOUT ZILXI
ZILXI is the first and only topical minocycline for adults with the pimples and bumps of rosacea. Applied once a day, the foam formula is gentle on already-sensitive skin while determined to help you take on rosacea.
ABOUT MINOCYCLINE
Minocycline has been shown to have anti-inflammatory effects. Many researchers believe this is why it helps treat the inflamed pimples and bumps of rosacea.
The way ZILXI works to treat rosacea is unknown.

ZILXI uses minocycline directly on the skin, from the top down.
ZILXI works by delivering minocycline directly on the skin, where it is absorbed. Unlike an oral medicine that is absorbed through the stomach into the bloodstream, ZILXI has a low rate of systemic side effects. The foam formula contains naturally moisturizing coconut and soybean oils.*
*ZILXI contains the following inactive ingredients: soybean oil, coconut oil, light mineral oil, cyclomethicone, cetostearyl alcohol, stearic acid, myristyl alcohol, hydrogenated castor oil, white wax (beeswax), stearyl alcohol, docosanol. ZILXI topical foam is dispensed from an aluminum container (can) pressurized with propellant (butane + isobutane + propane).
ZILXI Significantly reduced the PIMPLES AND BUMPS of rosacea IN CLINICAL TRIALS
60%-61%
Reduction
in the number of pimples and bumps by Week 12 (vs 49%-54% for placebo foam) in 2 clinical studies of patients with rosacea†
Some patients saw results as early as Week 4
49%-52%
of patients using ZILXI achieved
"Clear" or "ALMOST CLEAR"
skin by Week 12 (vs 39%–43% for placebo foam)
In a separate satisfaction survey of 890 patients with rosacea, the majority had a satisfying experience with ZILXI‡
†Trial design: Two 12-week, randomized, double-blind, vehicle-controlled (placebo foam) trials in subjects with inflammatory lesions of rosacea. A total of 1,009 subjects were treated with ZILXI and 513 with foam that did not contain minocycline, the active ingredient. Upon completion of the double-blind studies, 505 patients entered the open-label extension study for an additional 40 weeks of treatment with ZILXI.
‡According to the patient satisfaction survey at Week 12. Subject Satisfaction Questionnaire was completed at the Week 12 visit or at the final visit for those subjects who prematurely withdrew from the study. Percentages exclude missing responses. There is no clinical significance to qualitative survey findings. More than 60% of patients were satisfied or very satisfied. Patients were asked to describe their level of satisfaction based on a 5-point category scale from “very dissatisfied” to “very satisfied.”
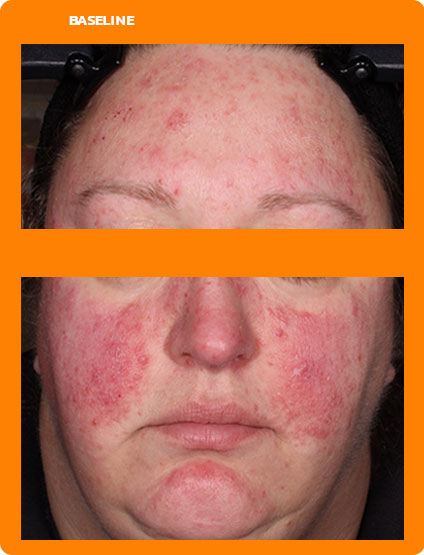
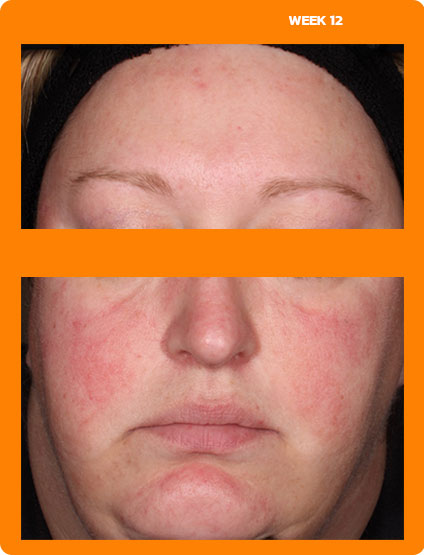
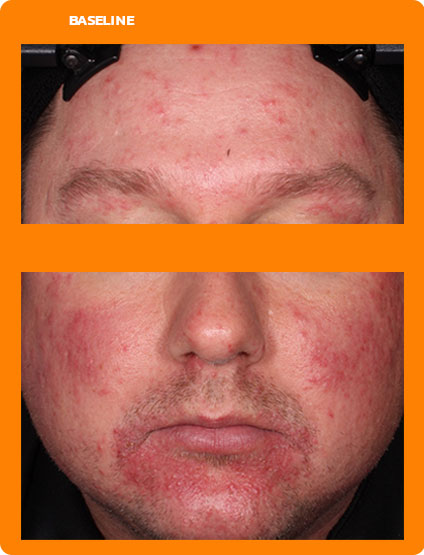
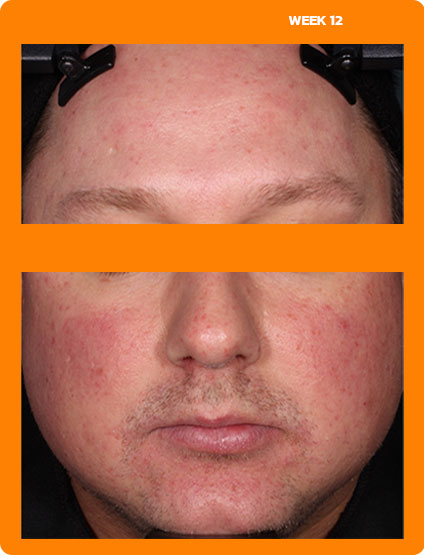
See real results from clinical trials
See visible skin improvements by Week 12.
Every person will respond differently to ZILXI. See clinical trial results above.
Proven Safe
-
The most common side effect reported more commonly than vehicle/placebo was diarrhea, in 1% of patients†
Well tolerated on already-sensitive skin
-
Long-term tolerability for up to 52 weeks of treatment†
†Trial design: Two 12-week, randomized, double-blind, vehicle-controlled (placebo foam) trials in subjects with inflammatory lesions of rosacea. A total of 1,009 subjects were treated with ZILXI and 513 with foam that did not contain minocycline, the active ingredient. Upon completion of the double-blind studies, 505 patients entered the open-label extension study for an additional 40 weeks of treatment with ZILXI.

HOW TO GET STARTED WITH ZILXI
See the tools and resources to help you save and start on ZILXI